A new scientific work led by Núria Torras and Elena Martínez, coordinator of the BRIGHTER project, shows a simple 3D bioprinting approach for the direct fabrication of advanced cell-laden tissue constructs by means of visible-light photopolymerization. The new approach allows the fabrication of cell-laden structures resembling the intestinal mucosa in a single printing step.
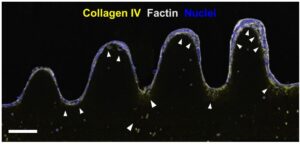
In recent years a lot of efforts have been made to produce gut engineered tissues representing reliable replicas of the in vivo tissue, including its particular architecture formed by finger-like protrusions called villi and invaginations called crypts. It is well known that the intestinal function relies not only on a healthy epithelium, but also on the stromal tissue (named lamina propria) that lays below. Therefore, to carry out consistent studies of inflammatory diseases, pathogen and microbiome interactions and even cancer, it is very important to have intestinal tissue models that resemble not only the epithelium but also the intestinal mucosa.
However, the intricate three-dimensional gut organization, together with complex biofabrication methods which entail a low cell survival rate and the high cost of some specialized equipment, limit the advances in the field of intestine tissue engineering. There is thus an urgent need to find easy fabrication methods of cell friendly complex structures, and in this scenario 3D bioprinting techniques can be a valuable tool to address this challenge, concretely light-based bioprinting techniques, due to their low cost, simplicity in use and versatility.
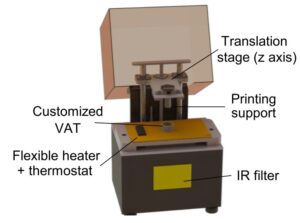
Researchers from the Institute for Bioengineering of Catalonia (IBEC) led by Núria Torras and Elena Martínez, researchers from BRIGHTER Project, have developed a novel approach to produce fibroblast-laden crypt-villous structures by means of digital light processing (DLP) stereolithography (SLA). The work is available as a pre-print on bioRxiv repository. This technique photopolymerizes layer-by-layer bioinks, which can include a suspension of cells. By employing an optimized bioink formulation and suitable printing parameters, they were able to obtain a robust biofabrication approach that yields functional gut mucosa, with an excellent cell viability rate, accurate spatial resolution and high printing throughput.
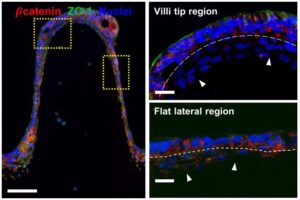
Researchers developed a customized DLP-SLA 3D bioprinting system for the direct printing of tissue constructs using transparent, soft hydrogels, by means of visible-light photopolymerization. With this new approach, they were able to fabricate, in a single printing step, cell-laden structures resembling the intestinal mucosa and presenting the 3D architecture of the small intestine, including villi and crypts and the epithelial and stromal compartments.
“We have proposed a light-based 3D bioprinting approach as a feasible alternative for developing in vitro cell culture models recapitulating the native microenvironment of the in vivo tissue, thus contributing on providing alternatives beyond the current state-of-the-art”.
Núria Torras, first author of the study.
This new bioengineered tissue is compatible with conventional testing techniques, and the cost-effective DLP-stereolithography approach offers scalability, good resolution and fabrication speed, being a very good alternative to the existing in vitro systems.
Reference article: A simple DLP-bioprinting strategy produces cell-laden crypt-villous structures for an advanced 3D gut model. Núria Torras, Jon Zabalo, Eduardo Abril, Albane Carré, María García-Díaz, Elena Martínez. bioRxiv